
In September, Keylights won the CIIF Innovation Award and obtained the MDSAP System Certificate.
Venus 100 Series product are exported to Nepal, India, Bangladesh, Oman and other countries.


In December, Alpha 550 Automatic Sample Pipetting System was officially approved and put into clinical use.
In September, the injection molding production line was put into use.
In August, Venus 100 Series Automatic Chemiluminescence Immunoassay Analyzer obtained the registration certificate and was installed clinically.
In August, Keylights (Chongqing) obtained the Production License.
In March, Two Major R&D Centers (Chongqing and Shenzhen) were established.
In March, the CNC Processing And Manufacturing Center was put into use.
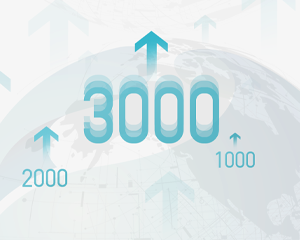
In December, the total products installed hit 3000 since the first analyzer installed on-site in 2016.

In December, STAR YB-6500 Sample Transportation System compatible with Keysmile's open chemiluminescence series was put into clinical use.
In December, Keysmile obtained the Certification Of Intellectual Property Management System.
In September, All series of Keysmile automatic chemiluminescence immunoassay analyzers won the honorary certificate of Outstanding Domestic Medical Equipment issued by China Medical Equipment Association.
In August, SMART 500 series products obtained CE admittance and began to be promoted in the European market.
In August, the Automatic POCT Detection Platform and the STAR YB-6500 Sample Transportation System was unveiled in the 17th CACLP.
In July, the Key Technology And Application Of High-precision Hypersensitive Immune Detection won the 1st prize of Chongqing Science and Technology Award.
In February, the All series of Keysmile automatic chemiluminescence immunoassay analyzers was listed in the Catalogue of Imperative Medical Equipments for the Prevention and Treatment of COVID-19 published by the China Medical Equipment Association.

In December, Keysmile was recognized as Chongqing Enterprise Technology Center.
In December, Keysmile was rated Top 10 Technological Innovation Enterprises in Chongqing Liangjiang New Area.
In October, Keysmile was honored as the Innovation and Entrepreneurship Demonstration Team.
In September, Keysmile Growth College officially opened with comprehensive courses covering machinery, electronics, software, biology, and other work-related fields.
In June, Keysmile products passed the International Safety Standard Test and obtained the TÜV SÜD certificate.
In June, SMART 6500 Automatic Chemiluminescence Analyzer was rated Major New Products In Chongqing In 2008.
In April, Automatic Magnetic Particle Chemiluminescence Immunoassay Analyzer SMART 500 series products obtained the registration certificate for sale;
In April, SMART 6500 series products obtained CE approval and began to be promoted in the European market.

In December, Keysmile was hornored with the Specialized And Sophisticated Enterprises That Produce New And Unique Products & Gazelle Enterprise.
In November, SMART 6500 went abroad and was successfully installed in Germany, Spain and other European countries.

In March, SMART 300 and SMART 3000S won the honorary certificates of Excellent Domestic Medical Equipment issued by China Association of Medical Equipment;
In March, the Chemiluminescence Immunoassay Analyzer SMART 6500 magnetic of particle series verified with biological experiments from reagent manufacturers on site at CACLP.
In February, Keysmile launched the Automatic Chemiluminescence Immunoassay Analyzer SMART 6500.
In January, the Automatic Chemiluminescence Analyzer won the honorary certificate of Chongqing High-tech Product.

In December, Keysmile obtained National High-tech Enterprise certificate.
In August, SMART 300 went abroad and was successfully installed in the United States.
In May, SMART 3000S microwell series completed the registration, and was successfully installed in the hospital in the same month.
In March, Automatic Chemiluminescence Analyzer SMART 300 was launched.







